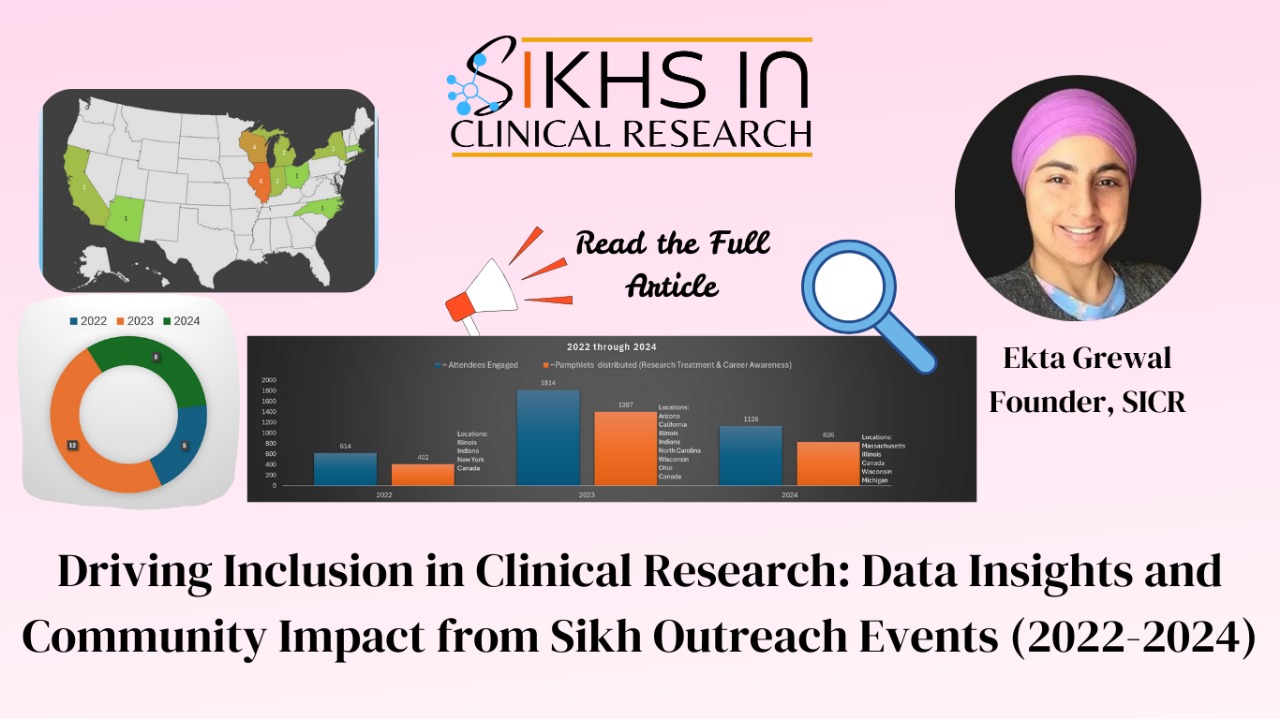
Over 3 million individuals participate in clinical research across the globe every year! This massive involvement in medical studies is driving the future of healthcare, but there’s one crucial element missing—diversity. For clinical research to truly benefit everyone, it’s essential that people from all backgrounds, including minority communities, are represented.
In today’s world of advanced medicine, we often hear about breakthroughs in treatments for cancer, heart disease, and even rare genetic disorders. But did you know that many of these groundbreaking therapies may not work the same way for everyone? The truth is, medical treatments aren’t one-size-fits-all, and that’s where diversity in clinical research becomes essential.
For years, clinical trials have predominantly involved participants from similar backgrounds, leaving out the voices and experiences of minority communities. This lack of diversity can lead to treatments that may not be as effective—or safe—for everyone.
By getting involved in clinical research, you’re not just helping yourself—you’re helping to shape the future of healthcare for your community and beyond. Let’s dive into why diversity in clinical trials matters and how we can all be part of this important journey.
How Diversity Fuels Innovation
When people from different ethnic, cultural, and genetic backgrounds participate in clinical trials, the results offer a more accurate picture of how treatments work in real-world settings. Diversity fuels innovation by allowing researchers to discover new ways that treatments can be adapted to work effectively across various populations. This leads to medical advancements that cater to the needs of everyone—not just a select few.
Reducing Health Disparities
Diverse participation in clinical research is key to addressing health disparities that disproportionately affect minority communities. Conditions like diabetes, hypertension, and certain cancers are more prevalent in some ethnic groups, yet these communities are often underrepresented in research. By stepping up and participating in clinical trials, you can help close this gap and ensure treatments are developed that meet the unique health needs of your community.
Opening Doors to Cutting-Edge Treatments
One of the most exciting aspects of joining a clinical trial is gaining early access to innovative treatments that aren’t yet available to the general public. For those with chronic conditions or diseases that have limited treatment options, clinical trials can offer a lifeline. It’s an opportunity to benefit from groundbreaking therapies while also contributing to the advancement of science.
Why Clinical Research Matters
Clinical Research Saves Lives: Every treatment available today started as a clinical research study. From revolutionary cancer treatments to breakthrough vaccines, the progress made in medicine has been possible due to clinical trials. Without diverse representation, the effectiveness of these treatments could be limited for certain populations.
Ask Your Doctor About Clinical Research: Ask your doctor if they are conducting any clinical research studies or if there are research treatment options available for you. Participating in a clinical trial could provide access to potentially life-saving treatments before they become widely available.
Benefits of Participating in Clinical Research
- Health Insurance Not Required: Many clinical trials offer no-cost treatments, making healthcare accessible to those without insurance.
- Compensation for Time and Travel: Participants are often compensated, making it easier for people from all walks of life to join clinical studies.
- Standard Procedures at No Cost: Participants can receive routine healthcare and testing at no cost.
- Access to Innovative Treatments: Participants can benefit from cutting-edge treatments not yet available to the public.
- Health Screenings at No Cost: Many studies provide comprehensive health screenings, offering insights into participants’ overall health.
Ensuring Safety and Informed Consent
When you participate in clinical research, you are fully informed about the study's purpose, risks, and benefits before making any decisions. A consent form ensures you understand every aspect of the study, and your participation is entirely voluntary. You can withdraw from the study at any time.
To ensure safety, institutional review boards (IRBs) review each study before it begins. Once underway, the research team monitors and supports patients throughout, safeguarding both patient well-being and data integrity.
Empowering Communities Through Knowledge
When minority groups participate in clinical research, they become empowered with knowledge about their health and the future of medical care. This knowledge doesn’t just benefit the individual—it ripples out to families and communities, creating a culture of awareness and proactive healthcare. By participating, you are helping to build a healthier, more informed future for generations to come.